Drag Labels to Explain Defect Might Affect Baby a Lungs
More About Materials Science
Defects
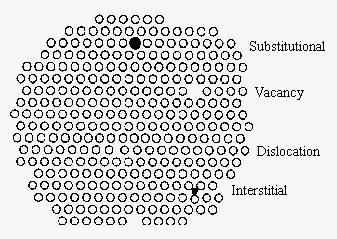
It is useful to think about solids in terms of a regular repeating blueprint of planes of particles. But it is important to recognize that solids are seldom perfectly ordered. There are four basic mechanisms for introducing a signal defect into the construction of a solid, equally shown in the figure below. When a particle is missing at one or more than lattice sites we get a vacancy. When a particle forces its way into a hole between lattice sites, nosotros get an interstitial impurity. Substitutional impurities result from replacing the particle that should occupy a lattice site with a different particle, such equally substituting a One thousand+ ion for a Na+ ion in NaCl. (If an ion with a different charge is substituted, the electric neutrality of the crystal must be maintained. If a Ca2+ ion is substituted for a Na+ ion, for example, a second Na+ ion must leave the crystal so that it doesn't pick up an electric charge.) Dislocations are 1-dimensional defects caused by holes that are not large enough to be a vacancy.
When a significant fraction of the original particles are replaced by impurities, information technology is possible to get a solid solution. Alloys, such as statuary and brass, are examples of solid solutions. Bronze is a solution of tin can dissolved in copper. Brass is a mixture of copper and zinc that tin contain as little as x%, or as much as 45%, zinc.
Distortions of the crystal lattice frequently occur when impurities are added to a solid. As a consequence, point defects oft determine the backdrop of a textile. They tin change the ease with which a material conducts electricity, its mechanical force, its power to be shaped by hammering (malleability), or to be fatigued into wires (ductility). Dissolving small amounts of carbon in fe, for case, give the alloy known every bit steel, which is significantly stronger than iron. But higher percentages of carbon brand steel so brittle that it can shatter when dropped.
Indicate defects misconstrue the lattice and provide a way for atoms to move most the solid. Atoms tin motility from a lattice site into a vacancy, for example, creating a new vacancy, as shown in the figure below.

Theoretical calculations of the ease with which one plane of atoms should slip over another suggest that metals should be much more than resistant to stress than they are. In other words, metals are softer than i would look. Metallurgists have explained this by assuming that metals contain defects that permit planes of atoms to slip past each other more readily than expected. This hypothesis has been confirmed by microscopic analysis, which shows dislocations that run through the crystal. There are two types of dislocations: border or spiral dislocations. An edge dislocation is an extra half plane of atoms that goes part fashion through a solid structure, as shown in the effigy below.

Imagine, for example, a single playing card inserted halfway into a deck of cards. The line formed by the inserted menu would be a dislocation line. The presence of a dislocation defect allows 1 plane of atoms to slip more easily over its neighboring plane of atoms, equally shown in the figure below. Not all the atoms in the 2 planes move past each other simultaneously; they move ane row at a time.

An frequently quoted analogy is that of moving a carpet. Dragging the carpeting beyond the floor is difficult because of the friction developed from the contact of the surface of the carpet with the flooring. Imagine what would happen, however, if a wrinkle is put into the carpeting, as shown in office (a) of the figure below. The carpet can now exist moved by pushing the wrinkle beyond the flooring, because only the friction between a small-scale section of carpet and the floor has to be overcome. A similar phenomenon occurs when ane plane of atoms moves past another by means of a dislocation defect.

Because they allow planes of atoms in a solid to movement 1 row at a time, dislocations can weaken a metallic. Paradoxically, they can also strengthen a metal when the dislocations intersect to production knots like to the intersecting wrinkles in the figure labeled "b" in the figure beneath. This phenomenon is encountered with metals that have been piece of work hardened. Consider what happens, for case, when a piece of atomic number 26 is heated, hammered, cooled, reheated, and reworked to form wrought fe. In the course of work hardening the metal, intersecting dislocations are generated that hinder the movement of planes of atoms.
Screw dislocations are more difficult to visualize than edge dislocations.The figure beneath shows how a screw dislocation is produced when 1 side of a crystal is displaced relative to the other side. For either edge or screw dislocations a distortion is produced around the dislocation with a corresponding stress produced within the material.

![]()
Metals, Semiconductors, and Insulators
A meaning fraction of the gross national product (GNP) of the United states, and all of the contribution to the GNP from high-engineering industries, tin exist traced to efforts to harness differences in the way metals, semiconductors, and insulators deport electricity. This deviation tin be expressed in terms of electrical conductivity, which measures the ease with which materials conduct an electric current. It can also exist expressed in terms of electrical resistivity, the inverse of conductivity, which measures the resistance of a fabric to conveying an electric accuse.
Silver and copper metallic are amid the best conductors of electricity, with a conductivity of only 106 ohm-cm. (This is why copper is the metal most often used in electric wires.) The conductivity of semiconductors such as silicon and germanium is 108 to 1010 times smaller. (When pure, these semimetals accept a conductivity of 10-2 to 10-4 ohm-cm.) Insulators include drinking glass (ten-x ohm-cm), diamond (10-14 ohm-cm), and quartz (ten-xviii ohm-cm), which all have an extremely small tendency to acquit an electric current.
The x24-fold range of electrical conductivity is non the but difference amid metals, semiconductors, and insulators. Metals become improve conductors when they are cooled to lower temperatures. Some metals are such good conductors at very low temperatures that they no longer accept a measurable resistance and therefore get superconductors. Semiconductors show the opposite behavior � they get much better conductors as the temperature increases. The difference between the temperature dependence of metals and semiconductors is so significant it is frequently the best criterion for distinguishing betwixt these materials. The large range of conductivities of solids is shown in the figure below.

The range of conductivities of solids span roughly 24 orders of magnitude.
Semiconductors are very sensitive to impurities. The conductivity of silicon or germanium can be increased by a cistron of up to 106 by adding every bit little as 0.01% of an impurity. Metals, on the other hand, are fairly insensitive to impurities. It takes a lot of impurity to modify the electrical conductivity of a metal by as much equally a factor of 10; and unlike semiconductors, metals become poorer conductors when impure.
To explain the behavior of metals, semiconductors, and insulators, we need to sympathize the bonding in solids in more item. Considering information technology is the lightest chemical element in the menstruation table that is a solid at room temperature, let'south start past edifice a model of what happens when lithium atoms interact. As a first step, we can consider what happens when a pair of lithium atoms with a 1southward ii twos 1 configuration collaborate to form a hypothetical gas phase Liii molecule. The Li2 molecule is formed by placing two electrons in the bonding domain between the two Li nuclei.
Now permit's imagine what happens when enough lithium atoms come together to form a piece of lithium metallic. The valence electrons are no longer confined to the region between pairs of lithium nuclei, as was the example for an isolated Liii molecule in the gas phase. In the metallic, each lithium cantlet is perturbed by its neighbors and the energy states of each atom are slightly altered. The 1s orbitals on the various metallic atoms collaborate to class a band of orbitals whose energy falls within a range from slightly beneath the energy of the isolated ones orbital to slightly above this energy, as shown in the effigy beneath. The same matter happens to the 2due south orbitals.

Each of the orbitals in these bands can concord ii electrons of reverse spin. Considering there were 2 electrons in each of the isouth orbitals that formed the lower-energy band, the "1s" band is filled. But there was only one electron in each of the iis orbitals that formed the higher-energy band, which means that the "2s" band is simply one-half-filled. It takes niggling, if any energy, to excite one of the electrons in the 2s ring from i orbital to another in the ring. (The energy gap between orbitals in the 2s band in lithium is only well-nigh 10-45 kJ.) By moving from orbital to orbital within the 2s band, electrons can move from ane end of the crystal to the other. This ring of orbitals is therefore called a conduction band because it enables lithium metal to conduct electricity.
Permit's now turn to magnesium, which has a [Ne] 3 due south 2 configuration. The 3s orbitals on the neighboring magnesium atoms would overlap to form a band of 3s orbitals. Because in that location are two electrons in each 3south orbital, this band is totally filled. The empty iiip orbitals on magnesium, notwithstanding, likewise interact to grade a band of orbitals. This empty iiip overlaps the 3south ring in magnesium, so that the combined band is only partially filled, allowing magnesium to acquit electricity.
The differences in the way metals, semiconductors, and insulators acquit electricity can be explained with the diagram in the figure below. Metals have filled bands of core electrons, such equally the 1due south band in lithium or the onesouth and 2s bands in magnesium. But they besides take partially filled bands of orbitals that allow electrons to motility from 1 end of the crystal to the other. They therefore acquit an electric current. All of the bands in an insulator are either filled or empty. Furthermore, the gap between the highest energy filled band and the everyman energy empty band in an insulator is then big that it is difficult to excite electrons from one of these bands to the other. As a upshot, it is hard to movement electrons through an insulator.
Semiconductors besides have a ring structure that consists of filled and empty bands. The gap between the highest energy filled band and the lowest energy empty ring is minor enough, however, that electrons can be excited into the empty ring by the thermal energy the electrons deport at room temperature. Semiconductors therefore autumn betwixt the extremes of metals and insulators in their power to conduct an electric current.

To sympathize why metals become better conductors at low temperature it is important to remember that temperature is a macroscopic reflection of the kinetic energy of the private particles. Much of the resistance of a metallic to an electric current at room temperature is the event of handful of the electrons past the thermal motion of the metallic atoms every bit they vibrate back and forth around their lattice points. Equally the metal is cooled, and this thermal motility slows down, there is less scattering, and the metal becomes a ameliorate conductor.
Semiconductors become ameliorate conductors at high temperatures because the number of electrons with plenty thermal free energy to be excited from the filled band to the empty ring increases.
To understand why semiconductors are sensitive to impurities, permit's wait at what happens when we add a small amount of a Group VA element, such every bit arsenic, to one of the Group IVA semiconductors. Arsenic atoms have ane more than valence electron than germanium and silicon atoms. Arsenic atoms can therefore lose an electron to form Every bit+ ions that can occupy some of the lattice points in the crystal where silicon or germanium atoms are usually found.
If the corporeality of arsenic is kept very modest, the altitude between these atoms is so big that they don't collaborate. As a result, the extra electrons from the arsenic atoms occupy orbitals in a very narrow band of energies that lie between the filled and empty bands of the semiconductor, as shown in the figure beneath. This decreases the amount of energy required to excite an electron into the everyman free energy empty band in the semiconductor and therefore increases the number of electrons that accept enough energy to cross this gap. As a result, this "doped" semiconductor becomes a very much better conductor of electricity than the pure semiconductor. Considering the electric charge is carried by a flow of negative particles, these semiconductors are chosen due north-type.
It is also possible to dope a Group IVA semiconductor with one of the elements in Grouping IIIA, such as indium. These atoms accept ane less valence electron than silicon or germanium atoms, and they can capture electrons from the highest energy filled ring to form holes in this ring. The presence of holes in a filled band has the aforementioned effect every bit the presence of electrons in an empty band � it allows the solid to carry an electric current. The electric charge is now carried by a flow of positive particles, or holes, and then these semiconductors are chosen p-type.

Bringing n-type and p-blazon semiconductors together produces a device that has a natural one-directional flow of electrons, which can be turned off by applying a small voltage in the opposite direction. This junction between n-type and p-type semiconductors was the basis of the revolution in industrial engineering that followed the discovery of the transistor by William Shockley, John Bardeen, and Walter Brattain at Bell Laboratories in 1948.
![]()
Thermal Conductivity
You lot may have noticed that metallic ice-cube trays feel significantly colder then plastic ice-cube trays when you remove them from the freezer. Your senses are apparently misleading you because the trays are at the aforementioned temperature � the temperature of the freezer. The metal trays feel colder because metals are much meliorate conductors of heat than plastic.
The ease with which metals behave oestrus is related to their power to conduct an electric current. Most of the energy absorbed past a metallic when information technology is heated is used to increase the charge per unit at which the atoms vibrate around their lattice sites. Just some of this free energy is captivated by electrons in the metal, which move from orbital to orbital through the conduction band. The net outcome is a transport of kinetic energy from one portion of the metal surface to another. Metals feel cold to the touch because the electrons in the conduction ring carry rut away from our bodies and distribute this energy through the metal object.
Plastics, on the other hand, are thermal insulators. They are poor conductors of heat because orbitals in which electrons are held tend to exist localized on an individual atom or between pairs of atoms. The but mode for electrons to bear energy through a plastic is to apply this energy to excite an electron from a filled orbital to an empty orbital. But the difference between the energies of the filled and empty orbitals is so large that this rarely happens.
The difference between thermal conductors and thermal insulators can be quantified by defining the thermal conductivity of a substance every bit the quantity of estrus transmitted per second through a plate of the material one centimeter thick and one foursquare centimeter in area when the temperature differential between the 2 sides of the plate is ane degree Celsius or ane Kelvin. The copper used to for pots and pans has a thermal conductivity that is more than than 5000 times the value for the styrofoam used for coffee cups, every bit shown by the information in the table below. This table is consequent with experience, which suggests that the air that gets trapped in the fibers of a downward-filled jacket is a better insulator than cotton, which is a much better insulator than nylon.
Thermal Conductivities of Various Substances
| Textile | Thermal Conductivity (J/southward�cm�M) a | Textile | Thermal Conductivity (J/south�cm�K) a | ||||
| Air | 0.00026 | Pb | 0.353 | ||||
| Drinking glass wool | 0.00042 | Cs 0.359 | |||||
| Cotton | 0.00057 | MgO 0.360 (100�F) | |||||
| Styrofoam | 0.00079 | Rb | 0.582 | ||||
| Carbon tetrachoride | 0.0010 | Fe | 0.804 | ||||
| White pine | 0.0011 | Li | 0.848 | ||||
| Oak | 0.0015 | K | 1.025 | ||||
| He | 0.001520 | C (graphite)b | 1.i-2.2 | ||||
| Paper-thin | 0.0021 | Zn | ane.16 | ||||
| Nylon | 0.0025 | Brass | 1.2 | ||||
| Water | 0.0061 | Na | i.42 | ||||
| Brick | 0.0063 | Mg | 1.56 | ||||
| Glass | 0.0072-0.0088 | Exist | 2.01 | ||||
| Concrete | 0.0086-0.013 | BeO | two.20 (100�F) | ||||
| Hg | 0.083 | Al | ii.37 | ||||
| SiC | 0.090 (100�F) | Au | 3.xviii | ||||
| NaCl | 0.092 (0�C) | Cu | four.01 | ||||
| ZnS (zinc blend) | 0.264 (0�C) | Ag | 4.29 | ||||
| Al2O3 | 0.303 (100�C) | C (diamond)c | 9.9-23.2 | ||||
| aAll values are at room temperature unless otherwise noted. bValue is dependent on the impurities in graphite and on the orientation of graphite, being larger in the direction parallel to the layers of carbon atoms. cValue is highly dependent on impurities and defects. | |||||||
![]()
Thermal Expansion
It is tempting to think near solids as if the particles were locked into position, the way bricks are used to build a wall. This would be a mistake, however, because the particles in a solid are in more or less constant motion � rocking back and along and rotating virtually their fixed positions in the crystal. This motion depends on two factors, the temperature of the system and the forcefulness of the interactions that hold the particles together. The higher the temperature, the faster the particles are moving. The stronger the forcefulness of allure between particles, the smaller the distances the particles move autonomously. Because the van der Waals forces that hold molecules together are much weaker than the bonds between atoms in a metal or betwixt positive and negative ions in an ionic compound, molecular crystal expand more when heated than metals or ionic compounds.
The divergence between the coefficients of thermal expansion of iron and copper was the source of a major problem for the Statue of Liberty, which consists of copper plates supported by an iron skeleton. The insulating material used to proceed these ii metals from coming into contact was inevitably rubbed away considering of differences in the rate at which these two metals aggrandize when heated and contract when cooled. (For each caste change in the temperature of the Statue, the volume of the copper metal changes past 40% more than the iron metal.) When this happened, the two metals came into contact, forming an electrical cell that greatly increased the rate at which the fe skeleton corroded.
The aforementioned phenomenon, however, is used to form the thermostats that turn electric appliances on and off. When two metals with very different coefficients of thermal expansion are joined to form a bimetallic strip, the metallic that expands the well-nigh when heated forces the adjoined metal strip to bend toward the metal with the smallest thermal expansion. This bimetallic strip can be used to brand a device that volition turn a heater on or off equally contact is made or broken with an electric contact, equally shown in the effigy below.

Thermal expansion and thermal electrical conductivity can work together to weaken a material. If heat isn't transported quickly through an object that is heated, i part expands more than chop-chop than another. If any cracks or flaws are nowadays, the hotter role of the substance will pull on the colder office and widen the cleft, causing breakage.
![]()
Materials Scientific discipline
Materials Science | Metals and Unit Cells | More about Materials Science | Ceramics
Periodic Tabular array If you are seeing this your browser does not support JavaScript. | Glossary | Cool Applets
Source: https://chemed.chem.purdue.edu/genchem/topicreview/bp/materials/defects3.html
0 Response to "Drag Labels to Explain Defect Might Affect Baby a Lungs"
Postar um comentário